We can enlist the help of
ultra-high-temperature nuclear reactors to do this rapidly and efficiently.
Climate change is caused by greenhouse gases, principal of which is CO2. Our developing economies use plenty of power, and the principal source of it is cheap fossil fuels: oil, gas, coal.
 |
| Beijing smog. |
More CO2 means that more heat is trapped within the atmosphere,
leading to increases in global average temperature. More heat also means more
extreme weather and faster swings in temperature and humidity, while the CO2
molecule’s reaction with seawater causes ocean acidification and a decrease in
its ability to absorb CO2.
Our immediate response to worsening
climate conditions is to burn more fuels. Air conditioning consumes a third of the peak power in US cities and the
number of air conditioned homes is increasing by the tens of millions every
year. Less developed countries that struggle with uncertainty in weather disasters
and crop yield double down on the most secure energy source currently
available.
 |
| Short-term relief, long-term pain. |
Alternative energy
sources, such as renewable energy or nuclear power, have their own issues.
Solar panels and windmills must be paired with expensive energy storage
solutions if they are to cover the needs of an electrical power grid. Nuclear
reactors have been developed to the point where they are the safest, cleanest
and most effective option, but only a handful of forward-thinking countries are
building the new generation of reactors, while others are denied them for
political reasons. How then to defeat the
self-reinforcing cycle of fossil fuel consumption that leads to worsening
climate conditions?
CO2 targets vs CO2 as the target
Punitive action has been put forward to reduce CO2 emissions. Carbon taxes, carbon quotas, carbon limits… they have varying results and can only realistically be burdened by the largest economies in the world. Smaller economies cannot afford to hamper their growth and development, especially seeing that they are only minor contributors to CO2 in the world.
 |
| Tesla cars have very good air filters but come at 3-5x yearly Chinese wages in price. |
Furthermore, no country, large or small, has the budget to
switch over to fully carbon-free economies within the next century. Imagine
every family being asked to buy a new electric car, every plane having to load
up on synthetic fuels, every factory torn down to be replaced with electrical
machines.
You might be willing to
and able to afford it. The richest companies in the world might have the budget
for it….but half the world’s population lives on $2.50 per day. And even if all this is
accomplished, it would not rid us of the damage already done to the
environment.
Unless a radical change in
economic structures and political motivations occur worldwide, controlling the
release of CO2 cannot be relied upon. Instead, we can take CO2
out of the atmosphere.
Most solutions require a
large quantity of precious metals to serve as catalysts, consume alkali metals
or simply try to hide it away in underground chambers. This is evidently unable
to deal with the 40+
gigatons of carbon dioxide we release each year.
 |
| Absorbing CO2 using soda/lime. |
A quick calculation
shows that just as many gigatons of calcium carbonate
would have to be found, or finding volumes greater than 20
trillion m^3 to store CO2 underground.
Furthermore, using grid
power to capture CO2 is a very expensive option. We rely on cheap electricity
to run our global economies and raising prices by up to +75%
will only push weaker economies to burn the cheapest energy source for power
(and strong economies to buy their power or produce their goods abroad with ‘dirty’
grids). Again, this is for simply capturing CO2 and not eliminating it.
A solar or wind-powered carbon
capture plant beats the energy balance problem but just does not have enough
power output to remove enough CO2 to make a difference. Each square meter of
solar panels would produce 30W/m^2
on average throughout a day. This is just enough to remove 7 kilograms per day
of CO2 from the air, if we take the capture cost in energy to be 370.8
kJ/kg. Not to solve the carbon problem, but just to contain it.
 |
| Solar thermal is more effective but still insufficient. |
Nuclear power can come to
the rescue.
Thermal decomposition
 |
| Construction of San Ofre Nuclear Generating Station began in 1964. |
A typical nuclear reactor
is over 40 years old. It has a multitude of uranium fuel rods that are packed
together in a large pressure vessel and their heat is used to boil water into a
steam at about 600K. A huge installation of turbines, generators and coolers
must draw water from a nearby source to convert that heated steam into
electricity. If the reactor fails, it fails spectacularly.
A 4th
generation or newer reactor would use fuel held in self-contained, hermetically
sealed graphite/ceramic ‘pebbles’. It reaches much higher temperatures – over
1000K – by using molten salts instead of steam. This allows it to be both more
compact and more efficient than before. If the cooling is cut off or the
reactor is breached, there is no chance of a meltdown or leak of radioactive
material.
While the question of why
we are not building as many of the new and improved nuclear reactors as
possible is straightforward and easy to answer, it is not the topic of this
post. Instead, we are concerning ourselves with the technology that allows for
the reactor to run at higher temperatures.
Some designs, such as Very
High Temperature reactors use helium as a coolant that will reach over 1500K. This
document
states that molten fluoride-salt coolant only fail at 1800K+. We know that the
pebble fuel elements themselves can survive more than 2800K.
Going further, we can look
at nuclear reactors meant for propulsion in space. They have the highest
temperatures because it would mean propellant is ejected at the highest
velocities, a key metric in determining rocket performance.
 |
| KANUTER |
We find that solid-core
nuclear thermal rockets had core temperatures of over 2800K, with the Project Timberwind
engines maintaining 3000K for several minutes. More recently, we have research
on uranium fuel held in niobium-carbides by engines that operate
at 3250K.
At the extremes, we have
reactors designs where the uranium is liquid. Designs such as
this heat
the coolant to 4,000K. Another NASA rocket design has spinning liquid
uranium held at 5,250K. It is not implausible to develop a nuclear reactor core
that operates at these temperatures.
What is the point of these
extreme temperatures for helping with climate change?
CO2 can be broken up into
carbon and oxygen. It is an energy-consuming process that is accomplished
naturally by plants using enzymes or artificially in certain catalysts. Neither
is a good option for our purposes, as they are slow and expensive methods.
We want to thermally
decompose CO2. At a high enough temperature, CO2 simply turns into a plasma
where carbon and oxygen ions dissociate freely. Efficiency is massively
increased, as every joule added to the plasma goes into breaking up chemical
bonds and any further heating just makes the reaction faster.
Thermal decomposition has
already been studied as an option for producing hydrogen from water. Oxygen is
reluctant to let go of its hydrogen atoms due to its electronegativity, meaning
that a lot of electrical power is required, but the task becomes much
easier to do at temperatures of over 2500K.
 |
| Thermal decomposition of water. |
You would not even need
electricity to split water once you reach 3500K. That is an important fact,
because producing heat is easy for a nuclear reactor, but turning it into electricity
requires turbines, steam and a power cycle that costs you 50 to 70% of the
reactor’s output.
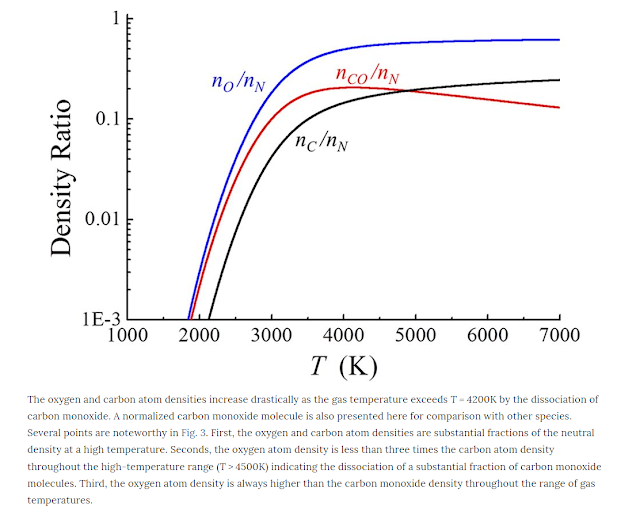
We find that at 3000K, 40% of CO2 molecules break up into CO and O
particles. The fraction becomes 50%
at 3600K.
Carbon monoxide has an even higher thermal decomposition temperature, beyond
3800K.
At 4000K, we can expect
that from every 1 mole of CO2, we get 0.15 moles of carbon, 0.5 moles of oxygen
and 0.2 moles of CO. Each mole of CO2 fully broken up requires to 530kJ. This
corresponds to 12 MJ per kg of CO2 that is decomposed.
Thankfully, nuclear heat
is in no short supply. Even small reactor cores can produce gigawatts of
thermal energy… indeed, most of the cost of a nuclear reactor comes from the
difficulty of containing the heat, not in producing it.
This sodium-cooled fast
reactor has a volumetric energy density of 300 MW/m^3. Rocket engine cores
are on another level. The Pewee reactor released 2.34 GW per cubic
meter, and the same page cites ‘advanced’ reactors having 40 GW/m^3.
The task and challenges
Hopefully, you are
starting to understand how ultra-high-temperature nuclear reactors and the
thermal decomposition of CO2 can go together.
If we manage to put CO2 in
a 4000K environment, we could be converting it into simple carbon and oxygen.
This directly eliminates the root cause of global warming.
Nuclear reactors are able
to put out gigawatts of heat and therefore process several thousands of tons of
CO2 every day.
Even better, the high
temperatures and plentiful heat can be used in many alternative ways. The most
obvious is to siphon off a bit of that power to produce electricity. Another is
to produce split water at similarly high temperatures, creating hydrogen that
can go towards
synthetic fuels.
The ‘catch’ is that no
usual material can handle these temperatures. The highest melting point ceramic
we know of is tantalum-hafnium-carbide (TaHfC) at 4488K. This is likely the only
material we can build the reactor walls out of.
 |
| Investigation of TaHfC's properties. |
Holding liquid uranium at
4000K inside a container that melts at 4488K is no easy task, but it is simpler
to accomplish than for the liquid core nuclear rockets cited above. We are not
worried about uranium flying out of a nozzle, nor do we need to transfer heat
quickly to a propellant. We also get a 488K temperature margin to lean on.
Another challenge is
preventing the radioactive products of uranium from contaminating the CO2 we
are trying to break down. Necessarily, the reactor accepts air from the
external environment and releases exhaust; we must make sure that we do not
contaminate the environment with fission products. This means a reactor design
made of multiple hermetically sealed vessels nestled inside of each other.
Finally, we have to manage
the gases and products entering and leaving the reactor. Air is composed not
just of CO2, but plenty of other things, such as water moisture and nitrogen. Only
pure CO2 should be accepted into the 4000K zone, and carbon must be separated
from oxygen before the gases cool down, otherwise the carbon would burn in the
oxygen and we’d get back the CO2 we started with. We cannot have the exhaust be
at a high temperature either, as it will react with the air and produce toxic
carbon monoxide or polluting nitrous oxides.
Proposed system
In this section, we
attempt to conceptualize a reactor design that solve the challenges mentioned
above. It is only one of the potential options we have for using extreme heat
to remove CO2 from the atmosphere.
It uses two key
components: a tantalum-hafnium-carbide container that doubles as heat
exchanger, and a supersonic expansion nozzle.
The container holds a critical
volume of liquid uranium at 4000K. TaHfC survives these temperatures without
the need for active cooling. The roof of the container would have channels for
CO2 to pass through and be heated. Having the hot gas vent into an open space
above the heat exchanger allow for further heating through blackbody radiation.
A black surface at 4000K emits 14.75 MW/m^2.
 |
| Vapor core nuclear power system operating at 4000K. |
For safety, the container
is held within ‘hot’ pressure vessel, itself separated from the environment by
a ‘cold’ pressure vessel. There are drain ports underneath the container to quickly
bring the uranium liquid below the critical point inside a TaHfC drain pool.
The nozzle is how carbon
is separated from the thermal decomposition products. Slowly cooling them would
allow oxygen to react with carbon monoxide and carbon to re-form CO2. Very
rapid cooling by using supersonic expansion through a nozzle could bring oxygen
below the ignition temperature needed to burn with carbon (680-1200K) or carbon monoxide
(880K). This implies a pressure drop of at least 6.7 times if we start at 4000K.
The carbon should condense at these lower temperatures and fall out of the gas
flow as a dust. For safety and efficiency, we will use a ten-fold expansion, in
addition to flushing the exhaust with cold air and using an oil spray to
separate the carbon dust from the oxygen.
Here’s how it would work
in sequence:
1) Extracting CO2 from the
air
We cannot use atmospheric
CO2 directly. It is mixed in with oxygen, nitrogen, water vapor and dust that
would not respond to the temperatures inside the reactor well.
The first step is
therefore to filter out the dust, cool the air to condense out water vapor, and
then liquefy CO2 by compressing it to over 25 bars at below room temperature.
The liquid CO2 rains out of the compressed gas and can be drained away.
2) Multi-stage heating of
CO2 to 4000K
We need to bring the high
pressure liquid CO2 up to 4000K.
To prevent thermal
stresses, we do this in multiple stages. The first stage might be to increase
the temperature from 300K to 400K, so that it becomes a gas again. Then, we
take it from 400K to 1000K, then 1000K to 1600K and so on. The start and finish
temperatures could be made to correspond to external thermal cycles, for
example to feed hot gases through a turbine. The final stage has the CO2 cross
from a stable state at 3000K, to the thermal decomposition temperature of 4000K
all at once.
This final stage takes
place in a heat exchanger built into the uranium container as described above.
3) Supersonic expansion
and extraction of carbon
The high temperature gases
leaving the heat exchanger are composed of carbon dioxide, carbon monoxide,
oxygen and carbon. They would be at a high pressure, perhaps 10 bars.
By passing the gases
through a nozzle, they can be made to expand very quickly. This ‘freezes’ them
before they have a chance to chemically react and recombine into carbon
dioxide.
An example of this is the
passage of a 4000K gas at 10 bars through a nozzle with an expansion ratio of
10. The gas exiting the nozzle would have its temperature reduced to 400K, its
pressure down to 1 bar and its velocity increased to about 2.3 km/s. The
transition from hot to cold would occur in less than a millisecond.
Oxygen would still react a
bit with carbon and carbon monoxide. To make this even less likely, we can add a
load of cold air to the exhaust stream. Adding mass to the exhaust slows it
down and maintains a below-ignition-threshold temperature. Adding 10 kg/s of
motionless air to 1 kg/s of supersonic exhaust can reduce the velocity by 90%.
It is best to spray oil
into the exhaust as well. This helps ‘rain out’ the carbon dust, much the same
way rain helps remove dust from the atmosphere. The oil would protect carbon
from attack by oxygen molecules and makes it easier to extract carbon dust from
the exhaust chamber as dust does not normally flow into tubes.
4) Exhaust treatment
The cold exhaust is either
recycled through the condenser of the first step or released into the
environment. If it is released, special precaution must be made to prevent
carbon monoxide from reaching the environment.
The oil laden with carbon
dust is filtered and dried to create a sort of powdered charcoal, or graphitized
using reactor heat to form storable bricks.
 |
| Graphite bricks. |
Graphite bricks can then be used in
construction, steelmaking, batteries or just stacked inside the empty coal mines
and oil wells we got all of our fossil fuels (and CO2 problem) from.
Funnily enough, coal miners could find new work as coal mine refillers...
A full-size diagram of
this process will be created in the next post.
Efficiency and performance
The source of all power in
this system is the nuclear reactor’s heat. It is consumed in three ways:
thermal decomposition of CO2, running the pumps and inefficiencies in heat
transport or isolation.
The thermal decomposition
process is not 100% efficient. Heating up CO2 from room temperature to 4000K
requires 4.4 MJ/kg. The thermal decomposition process itself requires 12 MJ/kg.
For every 1 mole of CO2 that goes in, we get 0.15 moles of untouched CO2 and
0.2 moles of carbon monoxide. This is an efficiency of 65%, which bumps up our energy
requirements to a total of 22.8 MJ/kg.
This is a lot!
However, it must be
compared to the raw heat output of a nuclear reactor. 1 GW of nuclear heat
would remove 3,790 tons of CO2 per day. This can be the output of a reactor
core that fits on a truck bed.
 |
| Power in small packages. |
In terms of uranium
consumption, this 162,400 tons per year with 5% enriched high burnup fuel (65
GWd/t), and perhaps just 7,200 tons per year with fuel reprocessing
allowing 90% burnup instead of 4%. Worldwide uranium production is at 66,500
tons.
If we want to compare it
to our existing
nuclear output, it is about 34x the thermal output if we assume 33%
efficiency (the figures are given in electrical output).
Improvements and alternatives
There are many ways to
improve on this design, or to do things differently.
-Gas recycling
The exhaust of the design
described above is depleted in carbon but still contains oxygen, carbon
monoxide and CO2. Recycling these gases into the reactor allows for a greater
thermal decomposition efficiency. If the exhaust chamber can handle it, 2.3
km/s gases can be directed back into the heat exchanger. As they strike the
exchanger’s surfaces, the gases compress and heat back up to a temperature
close to 4000K, which allows for a recycling of most of the heat energy lost to
the exhaust.
A reduction in energy
consumption and getting a higher fraction of CO2 broken down can bring the 22.8
MJ/kg requirement down closer to the 12 MJ/kg minimum.
-MHD separation
A supersonic expander can
be enhanced or replaced by electromagnetic separation of the carbon from the
thermal decomposition products. A magnetohydrodynamic device acting on a 4000K
stream of gases would even be able to generate electricity at high efficiency.
-Electric heating
Electricity produced using
an MHD device or a gas turbine siphoning heat from the reactor can be used to
further heat the CO2. We can both lower the material temperature requirement
down to the point where a solid core reactor is usable (3000K) and increase the
temperature that the CO2 reaches (4400K+).
The heating can take the
form of an electric arc, a laser or radiofrequency heaters.
-Reactor laser
An even more speculative heating
method is to use the reactor’s fission output directly
to produce a laser beam that acts upon the CO2.
-Pebble bed reactor
The unsurpassed security
of a pebble bed reactor could be achieved at 4000K if we solve the thermal
expansion issues that might arise if we held liquid uranium inside a TaHfC
shell.
The TaHfC would need great
strength to handle the pressure of an expanding uranium center as it passes
from solid to liquid phase. This implies a very thick shell, which hinders thermal
conductivity… but this is of lesser concern in a reactor sitting on the ground
when compared to a nuclear rocket.









If 40% of CO2 has been decomposed at 3,000K that seems A: pretty good and B: more achievable more easily than 4,000K. If you could build two or three 3,000K plants for the price of one 4,000K plant (given the more exotic ceramics required for 4,000K) that may be a more effective route.
ReplyDeleteI'd like to see a deeper exploration of these plants' electricity generation capabilities too. Having a CO2 decomposition plant that also supplies energy to the grid would be a major selling point. Or, rather, having a powerplant that also decomposes CO2.
The difficulty with operating at 3000K is that only 3% of the CO2 ends up as carbon. The rest is decomposed, yes but into carbon monoxide which is an even sturdier molecule than carbon dioxide.
DeleteI do agree that having 3000K materials are simpler and easier than 4000K, and you can bring the CO2 up to higher temperatures using electric heating, but that will depend on the cost of producing and using electricity vs using very refractory ceramics.
I think in reality, a mass push towards nuclear power makes electricity so cheap that using some of it to decompose CO2 using a laser, for example, would become economically acceptable as a sort of indirect consequence.
Surely CO and O could be persuaded to bond with some other substance at those temperatures (possibly with a catalyst)? By introducing something cheap into the hot gases you could create a slag as they react with it rather than going for a pure carbon output. With this you could run at a lower temperature, allowing cheaper construction and faster throughput. Harder slag-type materials can sometimes also be used in the construction industry instead of aggregate so you could also reduce quarrying as a bonus. I am no chemist though, so apologies for the lack of detail.
DeleteYou are correct, if you just exhausted the C and O into the air, it would burn and return to CO2. However, we added a supersonic nozzle to the reactor. It can help reduce the exhaust temperature from 4000K to 400K in under a millisecond. This 'flash-freezes' the C and O into solid carbon and gaseous oxygen respectively. As the exhaust falls below the ignition temperature of carbon and oxygen, we hope to avoid combustion.
DeleteWhat I meant was run the reactor at, say, 3000K and accept a much lower C content in return for a higher throughput. As you point out, the CO and the O will want to recombine but given the heat and a catalyst I was suggesting you could force it to react with a third substance to produce an inert slag instead of CO2. The result (if it is viable) would be to significantly reduce the energy required per kg of CO2 removed from the atmosphere and you would potentially have a byproduct you could sell in bulk.
DeleteIt might be a less elegant solution but they say perfect is the enemy of good, and cost is critical to the viability of the project.
That could be a good solution. The amount of 'third substance' you'd need would be huge however, and it has to be more reactive with carbon than oxygen is.
DeleteThat is really amazing approach. I'm currently writing on decarbonizing the industry and options with CO2 from a holistic view without details on technical issues. I find distraction of CO2 utilizing nuclear heat is key if adopted economically well. For the cases where C, CO and O2 is generated from the decomposition, CO after filtering out carbon can be sent to CO boilers to generate additional steam and power. Although it is combustion process but require less fuel. CO2 recycle can be establish back to the process. However, it needs to be look into a full flowsheet for energy balance and feasibility.
DeleteHi Mohammed.
DeleteI am glad you enjoyed the idea. The concept is physically valid, but the design I described is an 'emergency solution', to be used in case of catastrophic climate change that has to be solved as quickly as possible.
A more practical, near-term design would only heat the CO2 to about 1500-200K, and then do further heating to 3000K by an electric resistance heater
At that temperature, there are enough ions to consider it a conductive plasma. This means an electrical current can be used to perform efficient, high temperature electrolysis.
I think you may have made a mistake with your math regarding solar panels. If one square meter of solar panels generates enough energy to remove 7 kg of CO2 per day, then one square meter would be able to remove 2.555 tons per year. Thus, to offset 40 gigatons per year, 15,655,577,299 square meters of solar panels would be needed. That's a square approximately 125km across. It's a lot of solar panels, but not impossible. The current front-runners in the direct air carbon capture industry claim costs between $50 and $100 per ton. Converting that CO2 to a carbonate mineral using ultramafic rock like olivine would cost another $40-50 per ton. That would put annual costs at around $3-5 trillion annually. Alternatively, building 29 TW of nuclear would cost maybe $1-200 trillion at current costs for new construction. In either case, it makes more sense to give free nuclear or renewable electricity to poor countries rather than have them burn coal and then spend a bunch of money pulling out the CO2 from that coal. For certain applications, though, such as international travel, sequestration would be cheaper than converting every plane to hydrogen or biofuel. I just don't see a situation where any group of countries powerful enough to build out 29 TW of nuclear plants would not also be able to incentivize or bully the rest of the world into drastically cutting emissions first.
ReplyDeleteI fixed that mistake, thanks for spotting it.
DeleteThe cost of nuclear energy as planet-changing scales is necessarily cheaper than the reactors we produce piecemeal today, because of scale savings and also because we won't be associating them with all the equipment needed to turn reactor heat into grid electricity.
If we massively pushed nuclear power for grid electricity, then we could bring electrical costs down enough to make switching over from fossil fuels the economically preferable solution in all cases, and maybe even have enough left over to remove CO2 using something like lasers. Our electrical consumption goes up and down during the day and it is too expensive to store the excess capacity, might as well use it to save the planet during quiet periods of the day.
If we assume a mass buildout of 29 TWe of Nuclear Energy using standard international FOAK pricetags for the VVER-1200 and APR-1400 (the HPR-1000 is far cheaper but has yet to be exported), then a worldwide buildout of Nuclear reactors at $4300 per kWe would be 124.7 Trillion dollars.
DeleteSolar/Wind is far harder to calculate because you have to account for increasing costs as penetration increases, and the inverse square it follows isn't a perfect exponential curve. VRE costs double at 30% and then it's roughly every 10 to 15% penetration afterwards that they double again. Getting to 50% VRE should cost about the same as 50% nuclear though, not accounting for lifespans.
The comparison is not apt. Those price tags are for complete electrical power generating installations running at about 800K. The design I described is for a reactor core and gas pumps, a much smaller and simpler thing. Also, operating at 4000K means you get 5x the power for the same equipment than an 800K reactor, although the cost of TaHfC would means that it doesn't exactly translate to 5x cheaper.
DeleteAlso, those prices are for a handful of projects, kind of like building a few Ferraris. Trying to build at a scale that has a global impact would mean churning out nuclear reactors like Fords.
Because the first step of this process is getting pure CO2, the whole process proposed here is a solution to storing the CO2. It's a really good solution, but I expect policy makers would just prefer to pump it into empty oil wells to store it.
ReplyDeleteOr store it in facilities and use it later? Perhaps like this?
You would be exchanging a permanent solution to CO2 for the 60-fold energy requirement reduction that comes with simply storing it. As technology progresses, energy only becomes cheaper and the balance shifts towards the nuclear option.
DeleteUsing nuclear energy to solve global warming in a way that I did not expect. You never cease to amaze me.
ReplyDeleteThanks for the kind words.
DeleteIs it possible for the reactor core be a breeder, or are there limitations with breeding at high power densities?
ReplyDeleteYou can turn it into one if you have a separate blanket of thorium being rotated around the inner pit of liquid uranium. It is a complicated design though.
DeleteSuch reactors would be most useful for continued military and civil (Orion, fracking) bomb grade plutonium production in a mostly fusion economy.
DeleteFusion breeders tend to get too big.
If you run your Pu-extraction process on your blanket continuously, it is conceivable that the residence time of the produced Pu-239 should be low enough that bomb-grade plutonium can be produced.
DeleteGiven the advantages of lower critical mass, smaller resultant device size, and "renewability", Plutonium would be an optimal bomb material for small nuclear devices, such that those required for Orion pulse units or oil well bombs. The main impediment, the higher cost of Pu compared to U-235, would be less relevant in a fast breeder economy.
Orion would consume truly staggering amounts of fissionables. A launch to HEO would take 300-1000 pulse units. A fast launch to Saturn (with 100km/s delta-vee for outbound and return) would require ten times the number of pulse units. 2-10kg of fissionables may be needed per device, with HEU requirements being higher due to higher critical mass. This comes to a requirement of many (10s-100s) of tonnes of HEU or Pu per year for a modest exploratory program, and a correspondingly large fast breeder or enrichment program (given that Cold War US warhead production was in the 1000s-per-year range, this is marginally doable even without fast breeders). A spacefaring society based on nuclear pulse propulsion or Zubrin NSWR would require even more gargantuan amounts of fissionables.
Wow this is an awesome concept but making high temperature nuclear reactor might be a much bigger problem.
ReplyDeleteWhy not we convert the atmospheric co2 into supercritical co2 and homogeneously mix uranium fuel with it. This way we can make a much simpler reactor with even higher temperature than the vapor core reactor.
Also is there a way to produce power or any other product which can be sold. The actual problem with carbon negative systems is that it is not profitable so no one puts money in it. So can you think about something frome wich profite can be made.
Mixing uranium with CO2 causes the carbon exhaust to become neutron activated and filled with radioactive fission products like Cesium 137, Strontium 90 and Iodine 131. You don't want those contaminating the environment!
DeleteA nuclear reactor like this one can destroy CO2, create synthetic fuels like ethanol and produce electricity all at once.
Interesting scheme, ingenious work as always. Especially - if I've understood correctly - that it can deal with CO2 and generate energy/create synth feuls at the same. One question that doesn't seem to be addressed in the article - given you mention these reactors being produced in your scheme 'like fords', what do you think the safety implications of this kind of role out are? You mention 'failsafe' designs, but I assume that refers to accidents, not active attempts at sabotage or as targets for terrorism? Doesn't the risk factors of a proliferation on the level you propose imply a risk profile thats unlikely to be accepted in reality?
ReplyDeleteThere is always a risk of nuclear fuel being turned into weapons. It is a risk that is actually independent of the number or even presence of nuclear fuel plants, because getting a small quantity of uranium to turn into highly enriched weapons-grade material is much harder to do than getting a large quantity of uranium to turn into low-grade reactor fuel.
DeleteActive attempts at sabotage would not work well here because of the way the fuel is contained - it is practically impossible to get a reactor to hurt its environment by spreading radioactive particles or blowing up, so all you can do at best it get it to damage its turbines and go into failsafe mode (drop its pebble bed core into the containment pit).
The increased danger might come from the much larger uranium economy that would spring up to meet the demand for nuclear fuel. More mines, operated by more countries, and more uranium ore/fuel circulating worldwide increases the chances of some of it slipping through security gaps and falling in nefarious hands. That's something a bit outside ToughSF's topics of interest.
Appreciate the clarification, thanks!
DeleteIs it possible to use a closed cycle gas core? Can't imagine a open cycle gas core being a good idea in atmosphere.
ReplyDeleteYou could. It would be much more complex than a liquid core though.
DeleteCool story bro. But CO (alongside N2) is an extremely thermally stable molecule that survives 6000K+.
ReplyDeleteSee the literature on the hottest flames with O2 and O3 as oxidizer and C2N2 and C4N2 as fuel. The products are N2 and CO.
I used the data provided in the documents I linked to.
DeleteSorry for the late response. That article is about a miceowave plasma torch, which by definition is ionized. When energies are high enough for ionization they are waaaay more than enough for dissociation. You can't dissociated carbon monoxide in a solid reactor.
DeleteSome dissociation of CO does occur at the temperatures that the most refractory materials can handle.
DeleteInteresting idea. My issue is being able to do this economically, without gov't subsidies, and having this thing operate as a net loss, making it a gov't run program start to finish. I'm not sold on climate change as a serious issue, just another shift in the weather that's not out of place in the last few centuries. And the extreme weather appears to be on decline, so chew on that why don't we.
ReplyDeletehttps://realclimatescience.com/2019/02/hiding-the-decline-in-extreme-weather/
As well, there ultimately comes the time where *POOF*, we're passed the arbitrary limit where we don't need to be sucking CO2 out of the atmosphere. Great, mission accomplished. What now? Do we start playing around with the ecosystem some more? Remove some more CO2 than necessary? Dump some of it back into the environment? And if we're not careful, we could cause a lot of harm by removing too much. From some looking around, 150 ppm is too low to support plant life.
https://notrickszone.com/2013/05/17/atmospheric-co2-concentrations-at-400-ppm-are-still-dangerously-low-for-life-on-earth/
The weather will change, the climate will alter, the sea levels might rise or fall. It's gonna happen so gradually that we'll have time to adjust. This article I find fascinating, because unlike the uneducated morons in representative governments the world over, this outlines a clear solution, even if I think it's totally unnecessary.
I would stick to scientific studies instead of newspapers when seeking data on controversial topics. Also, the objective with these nuclear CO2-crackers would be to bring down CO2 levels down to previously acceptable levels and not eliminate it entirely.
DeleteRegarding plant life... CO2 has swung between 180 and 300ppm naturally over the course of the past 500,000 years, based on samples from ice cores. Those amounts allowed for Europe to be covered with forests, for the Amazon to grow to its full extent and so on.
https://www.co2.earth/co2-ice-core-data
If there is a material which:
ReplyDelete3g/m^-3 gives Ek=20Mj
can it do the same job?
Thanks again, MB. This is very interesting. I have some ideas and questions. (DISCLAIMER: I am not an engineer, so if the ideas/questions are rather naïve from a technical perspective, please excuse me.)
ReplyDelete1) Perhaps we should regard these as factories with CO2 as a feedstock, as opposed to CO2 recycling facilities.
2) That being said: what is the feasibility of converting the C into other useful C allotropes like graphene, nanotubes, etc.?
3) If the reactor design is “fast,” it would consume actinide waste products.
For a reactor this size, what would be the production amount of these non-actinide wastes?
Would it be feasible to have the facility process and encapsulate the non-actinide wastes in C?
4) If the facilities could be made floatable, would it be feasible to have them oxygenate and revive the anoxic marine “dead zones”?
5) Per: the World Nuclear Association (http://www.world-nuclear.org/nuclear-basics/global-number-of-nuclear-reactors.aspx) there are 454 operable civil nuclear power nuclear reactors around the world, with a further 54 under construction. How quickly could 29k of these facilities come on-stream? Also, as these are ~59x more than the current and planned number, where would they be optimally sited?
Cheers,
Keith Halperin
You are welcome.
Delete1) The primary objective is removing CO2, turning that CO2 into a profitable product is a useful but complex proposition.
2) The ability to turn carbon vapor into allotropes of carbon depends on our manufacturing techniques. They are not able to produce large quantities of specific forms of carbon in a reliable manner so far, but nothing prevents it being possible in the future.
3) Using the reactors to burn nuclear waste is a great idea! I did not know with certainty how much harder it would be to add this functionality to the reactors, or how much riskier they would be in case of a failure, so I left them out.
4) I am generally wary of trying to modify the oceans in significant ways, because we do not fully understand how it works and what consequences doing something like adding oxygen to it could have. We could, perhaps, create a massive increase in aerobic bacterial populations, or cause a swinging cycle of expansion/contraction of the phytoplankton population...
5) That depends entirely on how hard these reactors are to build, how profitable they are and how much public motivation there is to accept increased nuclear risk to remove CO2 from the atmosphere. We could complete them all in a few years if we really had to.
Much obliged, MB.
ReplyDeleteRe: the oceanic oxygenation of dead-zones: Agreed- we shouldn't try anything too massive right away. It should be feasible though to initiate a number of small, controlled pilot projects to determine feasibility...Also, it's possible that we might hit a meta-stable CO@ plateau that would tend to keep the CO@ level fairly constant at this new level despite our efforts...
Year 2100 Projections https://www.co2.earth/2100-projections
ClimateInteractive.org | Based on climate action pledges of UN member countries
After reading this over several times I realized you made no mention of perhaps the greatest change this system would have on the world: the installed base for converting CO2 would be almost twice the entire energy consumption of the Earth today.
ReplyDeleteProducing that amount of energy would change economics, politics, Great Power relationships (especially if the distribution of these reactors is asymmetric: The United States giving them out to allies would cause a massive change in the global order in one direction, but if the Chinese were to be building and distributing them, the changes would be quite different).
I would moderate that statement slightly: it would be a huge amount of *thermal energy*, not *electrical energy*. My assumption is that producing thousands of reactors cores is easier than adding the necessary power converting equipment to turn it all into electrical power...
Deletebut I agree, having all the power available would go a long way towards fulfilling our energy needs, enough to move beyond fossil fuels at least!
The thing is, the countries do not have to give them out. They can build small nuclear parks that generate terawatts with a small footprint and still affect the global CO2 concentration.
Construction Time for PWRs (https://inis.iaea.org/collection/NCLCollectionStore/_Public/42/105/42105221.pdf):
ReplyDelete"The construction time in Germany, France and Russia was around 80 months and in Japan, about 60 months. The envelope of 95% of the cases includes a range between 50 and 250 months of construction time."
Let's say we can reduce the Japanese rate by 90% to 6 mos. For 2.9E4 reactors, this converts to an ~1.5E4 work years of construction time. Let us further assume that 580 reactors/yr are being worked on simultaneously, or ~130% of the total number of reactors in existence (per the report). This would require ~50 years to accomplish, and even granting a 2.8% annual increase in production reduces it to 25 years. While possible, I consider it less practical than *Marc Z. Jacobson's proposal to generate 12 TW through renewables by 2050 (https://web.stanford.edu/group/efmh/jacobson/Articles/I/CountriesWWS.pdf).
Cheers,
Keith
*What Jacobson does not sufficiently consider is that 1.2 kW/person (for an ~10G 2050 population) is insufficient to provide a decent standard of living (https://plot.ly/~alex/2208.embed).
To crack CO2, you need reactor cores and wind tunnel, essentially. A power station has much more equipment than that, to convert thermal energy into electricity.
DeleteAnother complicating factor is that one power station can have multiple cores. Fukushima for example, had 6 cores built over a period of about 12 years.
Finally, an effort to globally affect CO2 levels in the atmosphere can reasonably be expected to be burdened by several countries. A production chain of thousands of reactors can also end up being much faster than a handful of expensive projects, especially if some countries handle the non-radioactive side (pressure vessels and turbopumps) while others provide the fuel and special materials.
I think it would be better to use the heat of nuclear reactor to produce synthetic fuel from CO2 in the air and water. So you could replace fuel from oil with synthetic fuel witch doesn't increase the CO2 in the atmosphere.
ReplyDeleteThat would be a great solution for today's world. However, there is a possibility that we end up in a situation a few decades from now where there is too much CO2 in the atmosphere for civilization to continue. We would have to actively remove it. Synthetic fuels, while great at reducing emissions, do not affect the amount of CO2 already released into the air.
DeleteIf you produce more fuel than demand than you can store it and effectively reduce the CO2. Fuel are easy to store, you could use land/fill/mine/deep tunnel to store it. You need 1kg of fuel per 3 kg of CO2.
DeleteOtherwise it would be far better to use a gas core nuclear reactor, at 50 000K the CO2 will be completely destroy, and because the reactor fuel is gas, so you could extract the fission products in real time and keep the transuranium elements (the long live radioactive waste) inside the reactor until they are consume completely.
Fuel would take the form of methanol or gasoline. There are not easy to store for very long periods of time, as they turn into vapor at room temperature and are easily set on fire. A graphite brick is much more stable.
DeleteA gas core reactor would more efficiently break up carbon dioxide but won't make the results (oxygen and carbon) disappear!
Hi Matter Beam, I am a fan of your blog and am wondering if you ever might like to consider writing fiction and if so... Might you be interested in the prospect of literary representation into major trade publishing? To tell you a little bit about our literary agency: tridentmediagroup.com
ReplyDeleteTrident Media Group (TMG) is one of the world’s leading, largest and most diversified literary agencies. TMG represents over 1,000 bestselling and emerging authors in a range of genres of fiction and nonfiction, many of whom have appeared on the New York Times Best Sellers List and have won major awards and prizes, including the Pulitzer Prize, the National Book Award, the National Book Critics Circle Award, the P.E.N. Faulkner Award, the P.E.N. Hemingway Award, The Booker Prize, and the L.A. Times Book Award, among others.
Bio: Mark Gottlieb is a highly ranked literary agent both in overall volume of deals and other individual categories. Using that same initiative and insight for identifying talented writers, he is actively building his own client list of authors in fiction and nonfiction. Mark Gottlieb is excited to work directly with authors, helping to manage and grow their careers with all of the unique resources that are available at leading literary agency, Trident Media Group. During his time at Trident Media Group, Mark Gottlieb has represented numerous New York Times bestselling authors, as well as award-winning authors, and has optioned and sold numerous books to film and TV production companies. Mark Gottlieb is actively seeking submissions in all categories and genres and looking forward to bringing new authors to the curious minds of their future readers. https://www.tridentmediagroup.com/agents/mark-gottlieb/
I look forward to hearing from you, thanks.
All the best,
Mark
Mark Gottlieb | Literary Agent | Trident Media Group | MGottlieb@tridentmediagroup.com
355 Lexington Avenue, Floor 12 | New York, NY 10017 | (212) 333-1506 | tridentmediagroup.com
I will consider it, thanks for reaching out.
DeleteIs it possible to take this concept and apply it to fusion tractors?
ReplyDeleteWhat are fusion tractors?
DeleteCan this concept be applied to fusion reactors?
ReplyDeleteYes, and even more easily than with fission.
DeleteThermal decomposition involve the use of heat to Split a single compound into two or more simpler substances.
ReplyDeleteThermal decomposition reactions are usually endothermic as they require heat. Example is the thermal decomposition of Limestone(Calcium carbonate CaCO3
CaCO3----->CaO+CO2
That's right. That is why we need a nuclear reactor to provide the heat.
DeleteThere are both natural and human sources of carbon dioxide emissions. Natural sources include decomposition, ocean release, and respiration. chemistry analysis
ReplyDeleteThere are both natural and human sources of carbon dioxide emissions. Natural sources include decomposition, ocean release, and respiration. chemistry analysis
ReplyDeleteIn the link http://carbon.atomistry.com/decomposition_carbon_dioxide.html
ReplyDelete57.7% is indicated at 0.001 atm and 2000 ° C, which is something that can be achieved with the combustion of H2 + O2, or it can be achieved with a concentration of solar irradiance. Why not look for this alternative?
That is a good option. However, if we do go through a climate crisis and our civilization becomes dependent of the removal of CO2 to continue, then ultra-high-temperature reactors are our fastest and most effective method.
DeleteBy the way! You pose a very interesting dichotomy. Today, in the now immediate, our civilization and our existence does depend on eliminating the current enormous levels of CO2. His idea, by the way, is a way not to depend on CO2, however, we also have to take care of the current problem that is to decrease atmospheric CO2 concentrations
ReplyDeleteToday, we still have the ability to reduce our CO2 output and let natural systems slowly absorb the excess. The biggest carbon sinks, which are the oceans and the forests, take time to have an effect.
DeleteIf we do nog reduce our CO2 output, temperatures will continue to increase faster and faster, too fast for natural carbon sinks to work. The only solution would be something like these nuclear reactors.
Hi BM,
ReplyDeleteWe've invented a solar concentrator and the manufactured PoC is attaining temperature of above 2200 Degrees C.
We envisage powder ratings of about 1/2 MW should be possible.
A critical review for co2 decomposition using our apparatus will help our attempt to contribute to mitigate GHGs.
Kindly Oblige
Regards
Rajesh Jain
CEO,
High Temperature Solar Technologies
India
Dear Rajesh,
DeleteI am not an expert in this field, but I can tell you that at 2200°C, you can expect to thermally decompose 26% of the CO2 at 1 bar pressure, and 46% at 0.1 bar pressure.
Another piece of technology to separate the carbon from the other products would have to be developed.
In my opinion, it is more interesting for you to use your concentrated solar heater to make very efficient electrolysis of water, of over 90% (https://en.wikipedia.org/wiki/High-temperature_electrolysis). That is a demonstrated technology.
Regards,
Malik (Matterbeam)
What about using the heat to produce light and growing plant/vegetation with the light that would store carbon dioxide and of course produce us food as well
ReplyDeleteThe problem with growing plants is that their efficiency is 1-4% and they are limited to about 0.03 kg/m^2/day. That is too slow and inefficient for the rapid elimination of CO2.
DeleteOxygen is paramagnetic. You could use mass spectrocospic techniques to faster separation oxygen from other mass fragments on an expandable plasma flame...
ReplyDeleteHello, I am a physics uni student from Germany and have been reading your blog on and off for about a year. I finally got around to read this article, and had a few suggestions.
ReplyDeleteFirst, since we are already compressing and cooling the air to extract CO2, why not also extract argon. This argon can then be injected as a liquid, into the exhaust stream. Since there is about 23 times more argon than CO2 in the air, diluting the exhaust by 23 times with a liquid that vapourises, should serve to cool it down rapidly. Furthermore, the Argon acts to rapidly dilute the reactive species, and is inert. Argon does however have a rather low specific heat (although this is counteracted by its high molecular weight). If the argon is not sufficient, then we can do the initial cooling with argon, and only then inject regular air, once the exhaust has already been cooled some of the way. This allows the air to be injected at a temperature where it will be less reactive with the reaction products, but also with itself (reduce the production of those nasty nitrous oxides)
My second suggestion is to look into the use of membrane gas separation, on the input air. A quick google search seems to suggest that the technology isn't quite there yet, in terms of directly concentrating CO2 from the atmosphere, however I do know that these membrane devices are commonly used to provide oxygen and nitrogen supplies (albeit of mediocre purity) in a lab setting. What would be particularly useful, would be to somehow scrub out the oxygen, before the CO2 is extracted. This reduces the amount of gas needed to be compressed and cooled by 21%, effectively increasing the CO2 concentration by 26%, but also allows for an easier separation of the Argon, as Argon has a boiling point right below Oxygen (although I don't know how this changes with high pressures). The oxygen-depleted air can then be injected into the exhaust stream, with the advantage that there is no further addition of oxygen to the products. If combined with the argon injection, it is preferable to do after the argon, so that less nitrous oxides are produced, at these lower temperatures.
Lastly, I want to talk about location. The best place to put this, would be in a cold arid desert, such as Antarctica. In those conditions, the atmosphere holds less water, which means less of it has to be scrubbed out, and furthermore, there is no nearby human population to bother with the Terrawatt nuclear park. Alternatively, we can also choose an arid hot desert, that is not too far from the coastline. Once the necessary CO2 removal has been done, we can convert these reactors to be used for thermal desalination of water. We can use them to provide water to these arrid regions on a massive scale; something that may become even more needed due to climate change.
I have not yet run any numbers on these ideas, so don't be surprised if they don't end up working out.
Thank you for your many articles, my reading has been captivating so far.
Emil.
Thank you for reading these posts!
DeleteConcentrating the CO2 beforehand and using argon as coolant are brilliant ideas! Thanks.
While Antarctica would be the best place to put them, it should also be considered that your output is gigatons of solid carbon. Additional infrastructure would be needed to collect, transport and store all that output. Ideally, somewhere that won't see much tectonic activity but also not too far from existing infrastructure (roads, engineers, tool factories ect,) to help manage the carbon and the reactors.
You can use the reactors for desalination easily. Perhaps only expand the cleaned up air to 300°C, and have seawater do the rest of the cooling, as a form of waste heat scavenging. It will boil, and produce fresh water.
How do molten salt reactors based on thorium fit into the extraction of CO2 from the atmosphere?
DeleteThey would not be able to reach the required temperatures to directly break down CO2, because the molten salt would start to boil instead.
Delete"only a handful of forward-thinking countries are building the new generation of reactors, while others are denied them for political reasons."
ReplyDeleteUm... did you miss the memo on the fact that nuclear reactors are the most expensive form of electricity generation around?
Nobody in their right mind would say that France is denying nuclear reactors for political reasons but they are phasing it out in favor of wind and solar because it's many times more expensive. The French court of audit (similar to the US congressional budget office) found that the full lifetime cost of nuclear power for France was about 50 cents per kilowatt hour.
"Furthermore, no country, large or small, has the budget to switch over to fully carbon-free economies within the next century"
Dude. In 2019, 75% of global electrical capacity generation additions were from renewable energy sources. Renewable energy additions continued to rise through the pandemic even as fossil fuel additions slowed. Renewables are the cheapest form of electrical generation in history. And the costs for renewables keep falling. We're already seeing prices around 1 cent a kWh in Saudi Arabia and Morocco... 1/50th the cost that France saw with nuclear. And France is supposed to be the success case.
Elon Musk is offering $100 million Xprize for carbon capture. You should go for it!
ReplyDeletehttps://www.xprize.org/prizes/elonmusk
Why not just use high temperature concentrated solar power? The sun is a blackbody at around 5700k, just need to get the optics right.
ReplyDeleteThe concentrator optics necessary to reach 4000K are immense. You'd need to focus 14,500 m^2 of mirror area onto 1m^2 of receiver-absorber to reach that temperature.
DeleteWhat's worse is that your efficiency sharply decreases due to re-radiation losses. Your source of heat (the Sun) is a 5800K body adding 64.16 MW/m^2 into space. Your receiver meanwhile is radiating away at 14.5 MW/m^2. You can only add the difference, 49.66 MW/m^2, which is an immediate 22% efficiency loss.
When you factor in factor in realistic losses, like 90% reflective mirrors, atmospheric absorption, the solar capacity factor of 13% to 19% in the US...and other factors, you are probably going to end up with each 1 GW CO2 cracking installation requiring about 9 million m^2 of mirrors.
In fact, to meet the entire 29 TW need to crack all the CO2 produced annually, we'd be looking at 261 billion m^2 of mirrors.
Have to question your initial premise. More energy is used for heating than cooling, and that's not expected to change until at least 2060. So every degree of warming currently _reduces_ energy requirements.
ReplyDeleteenvironment of the United States
ReplyDeleteThe United States emits 17 tons of CO2 per capita. 3100950 km² of United States territory is covered with forests. and woodland accounts for 32% of the country's total land. The number of road motor vehicles per 1000 people in the United States is 200.
Some interesting information but as a retired academic chemist I must point out that there are simpler chemical methods of capturing carbon dioxide. Also Carbon monoxide is a useful chemical feedstock already use for example to make methanol. Carbon dioxide reacts with phenolic compounds again a useful chemical input for industrial use.
ReplyDeleteYa know speaking of Co2 we could also use the heat of nuclear rockets to enchant the ISP and thrust of Co2 gas thrusters for maneuvering in combat or we could use stuff like Arc jets or such propulsion
ReplyDeleteProbably not. CO2 thrusters are light and simple and cheap, and nuclear reactors tend to be the exact opposite. Furthermore, depending on what kind of temperature you're talking, you get carbon (nasty sooting) and oxygen (reactive, especially so at reactor temps). You could use another gas to get rid of sooting and reactivity, but then it's just an NTR. If you're looking for a combat NTR propellant, might I suggest hydrazine? It's dense, storable, and exothermically decomposes, and it allows you to share it with hypergolic missile propellants like MMH/NTO (and as they say, amateurs talk tactics, professionals talk logistics)
DeleteThe LANTR concept where oxygen is injected into the exhaust of a nuclear rocket is really interesting for combat applications.
Delete